With the growing global prevalence of dementia and continuing failures in Alzheimer’s drug trials, there has never been greater urgency to develop a platform that will help to embrace the wider complexity of systems and processes at play within a healthy brain to understand what sets it along the path of neurodegeneration.
The amyloid cascade hypothesis, which presumes the deposition of the amyloid-ß (Aß) peptide in the brain, is the main event triggering Alzheimer’s disease, was formulated in the early 1990s by one of our key project team members, Prof. John Hardy. This influential hypothesis has underpinned and influenced most research and drug trials. Nearly 30 years on, Prof. Hardy and others now believe the hypothesis is too simplistic, which has contributed to failures of drug trails aimed at reducing the amount of Aß in the brain.
It’s not that the hypothesis is wrong, it’s just that more information needs to be integrated and we need to move away from its linear concept. The direct implication of the current amyloid cascade model is that reducing levels of Aß in the brain would lead to reduced symptoms of Alzheimer’s, such as cognitive impairment. This has not happened, and so our project is considering three of the ways the model is oversimplified:
- Time: it is clear the disease process begins, probably with Aß deposition some 20-30 years before dementing symptoms become clinically evident
- Spread: the disease begins in one brain area, usually the hippocampus, and then spreads, possibly by a templating process in a non-random pattern throughout the brain
- Tissue, not just neurons: the model as drawn implies that this is a neuron-only process. Genetics led cellular biology is increasingly pointing to the importance of other cells types, especially microglia as also being crucial to pathogenesis.
Adding the critical component of time would enable the dynamic process of neurodegeneration to be studied in a model of Alzheimer’s, to understand at which point in the disease progression a potential drug therapy (or therapies) would need to be introduced.
Our project
Developing a 3D Dynamic Tissue-Level Model of Alzheimer’s Disease
Although Alzheimer’s disease has been defined in terms of a tissue pathology since it was described at the turn of last century, causes and treatments have been focused at the molecular level, and as yet no single link between the tissue and molecular scales has been found. In more recent times, as increasing quantities of data have been collected, a growing level of complexity has become identified in the disease and the realisation of a growing number of interrelated cellular and tissue components.
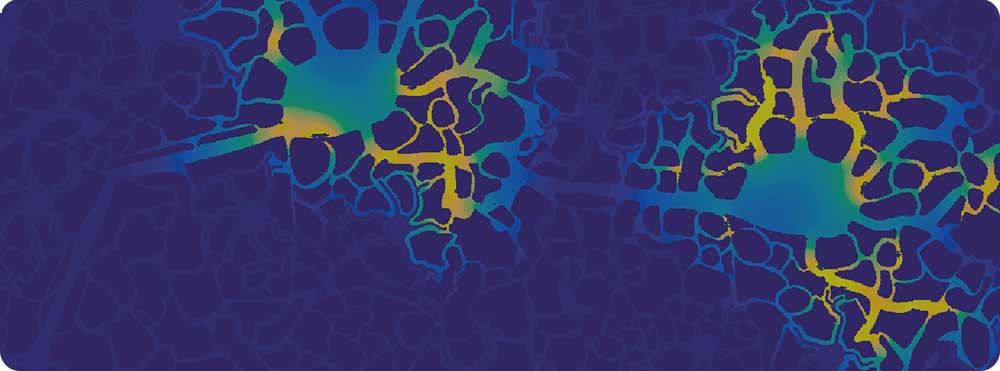
Our research aims to collaboratively begin the process of building a more holistic model of Alzheimer’s disease that starts to embrace its complexity. We are building towards a tissue model in which perturbations of the processes can be tested for their effects on outcome, enabling us to understand what tips the brain from health to disease, and then back to health again.
A pilot study of our research was presented at the world’s largest meeting of Alzheimer’s researchers at the American Alzheimer’s International Conference (AAIC 2017) in London last July (see ‘Now for some science …’ further down this page to view our poster and results of our pilot 2D model).
Key project team
We are a multidisciplinary team of geneticists, neuroscientists, physicists and engineers from University College London (UCL), which is recognised as a world-leader for its excellence in neurology and dementia research, innovative thinking and multidisciplinary collaboration, Rothamsted Research, which is a global leader in computational modelling and interdisciplinary research, and Glasgow University. Our key team members (from left to right) are:
- Professor John Crawford who is a theoretical physicist, world-renowned for his mathematical approaches to understanding the multi-scale biophysical organisation of living systems
- Professor Sir John Hardy who has made some of the most significant contributions in the field of Alzheimer’s research, including his formulation of the amyloid cascade hypothesis that has underpinned basic science and treatment research into Alzheimer’s since the early 1990s
- Dr Xiaoxian Zhang who is a highly experienced engineer and computational modeller with research interests in the use of tomography imaging and numerical modelling to study the flow and reactive transport in diverse fields of biological systems
- Me(!), Dr Jenny Rollo, a research scientist, carer, and developer of Decoding Alzheimer’s, who brought this multidisciplinary team together to help understand the complex mechanisms of Alzheimer’s through a new way of thinking (read more about my research profile here).
— Prof. John Crawford
— Professor Sir John Hardy

